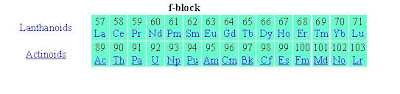
The f-block of the periodic table of the elements consists of those elements whose atoms or ions have valence electrons in f-orbitals. Actual electronic configurations may be slightly different from what is predicted by the aufbau principle. The elements are also known as inner transition elements. There are two series. Elements of the series in which the electrons are in 4f orbitals belong to the lanthanoid series. Elements of the series in which the electrons are in 5f orbitals belong to the actinoid series. There is a long-standing controversy as to whether La and Ac or Lu and Lr should belong to the f-block. IUPAC has now compromised by putting all four elements into the block, but this is contested, because there can only be 14 elements in f orbitals, so the block cannot be 15 elements wide.
All elements in the lanthanide series form M3+ ions. In aqueous solution the early lanthanides are surrounded by nine water molecules while the later lanthanides have a coordination number of 8. Cerium also forms compounds with the +4 oxidation state; Ce4+ has the very stable electronic configuration of the noble gas Xenon. Ce(IV) is a strong oxidising agent. Eu2+ has the configuration [Xe]4f7 and is a strong reducing agent. The existence of Eu(II) is attributed to the stability of the half-filled f-shell.
The lighter actinides (uranium to americium) show oxidation states of +3, +4, +5 and +6. The later actinides resemble the lanthanides in that the +3 oxidation state is favored.
The lighter actinides (uranium to americium) show oxidation states of +3, +4, +5 and +6. The later actinides resemble the lanthanides in that the +3 oxidation state is favored.